Introduction: Classical Hodgkin lymphoma (cHL) is characterized by immune dysregulation, with recurrent amplifications of the 9p24 locus encoding several genes including CD274 (PD-L1), PDCD1LG2 (PD-L2), and JAK2, frequent genetic and epigenetic downregulation of major histocompatibility complex (MHC)-I and (MHC)-II, and a tumor microenvironment (TME) defined by T-cell infiltration and high rates of PD-L1 expression. PD-1 blockade is highly active both as single agent and in combination with chemotherapeutic agents in relapsed/refractory (RR) cHL. However, the mechanism by which PD-1 blockade promotes clinical responses in combination with chemotherapy, and whether the mechanism of action varies based on tumor or TME-specific expression of either MHC or PD-L1 remains unclear.
Methods: We previously conducted a phase II study in patients with relapsed or refractory cHL evaluating pembrolizumab in combination with gemcitabine, vinorelbine and liposomal doxorubicin (P-GVD) before proceeding to high-dose therapy and autologous hematopoietic cell transplantation (AHCT) (Moskowitz AJ et al, JCO 2021). Treatment consisted of 2 to 4 cycles of P-GVD followed by AHCT. We performed combined single-cell surface protein identification, RNA sequencing, and (T cell receptor) TCR sequencing using the 10x Genomics Single Cell Immune Profiling Platform on paired C1D1 and C3D1 peripheral blood mononuclear cells (PBMCs) from 23 patients who received P-GVD and 5 control patients with relapsed cHL treated with standard chemotherapy regimens. Expression of MHC-I, MHC-II, and PD-L1 on both Hodgkin Reed Sternberg (HRS) cells and within the TME was assessed by immunohistochemistry.
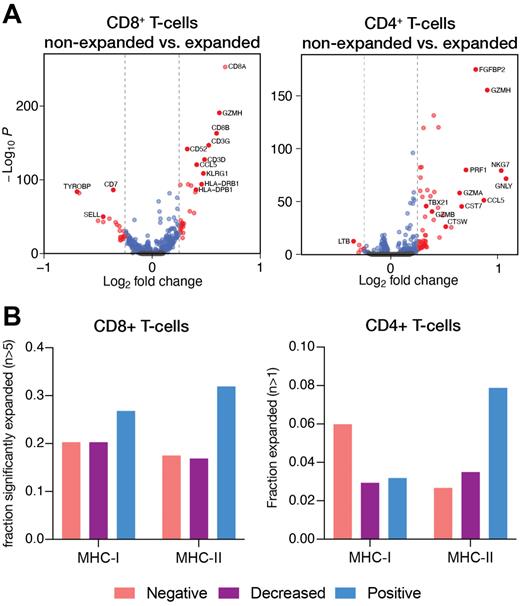
Results:Of 38 evaluable pts, the complete response (CR) rate after 2 or 4 cycles of P-GVD was 95%. After a median follow-up of 40 months (range: 2-56), only 1 pt experienced relapse 23 months after AHCT and another pt died 41 months after AHCT while in remission due to unrelated causes. The estimated 40-month progression-free survival (PFS) is 96% (95% CI: 91-100). CITE-Sequencing of PBMCs across 56 samples from 28 patients revealed predominantly CD4+ T, CD8+ T, NK, and myeloid cells within the peripheral blood, with smaller proportions of hematopoietic progenitors and plasmacytoid dendritic cells. Combined RNA and TCR analysis of the T-cell compartment revealed significant pre-treatment clonal expansion of both CD4+ and CD8+ T-cells exhibiting a cytotoxic phenotype, including expression of GZMH, KLRG1, and CCL5 by CD8+ T-cells and expression of PRF1, NKG7, GNLY, FGFBP2, and GZMH by CD4+ T-cells ( Fig. 1A). Correlation of pre-treatment clonal expansion with tumor-specific MHC status demonstrated that clonal expansion of CD8+ T-cells was more robust in patients whose tumors expressed either MHC-I or MHC-II; conversely, clonal expansion of CD4+ T-cells was most robust in tumors expressing MHC-II but lacking MHC-I, suggesting cytotoxic CD4+ T-cell responses may promote anti-tumor immunity in MHC-I-deficient tumors ( Fig. 1B). Both CD4+ and CD8+ T-cell clonal expansion was highest in patients whose tumors expressed high levels of PD-L1 on stromal cells. Treatment with P-GVD did not result in further clonal expansion of either CD4+ or CD8+ T-cells but did increase expression of cytotoxic genes in pre-treatment expanded CD4+ T-cells, including NKG7 and PLEK, while downregulating negative genes of T-cell function in pre-treatment expanded CD8+ T-cells, including TNFAIP3 and DUSP1, suggesting that P-GVD promotes anti-tumor immunity in cHL by enhancing the cytotoxicity of tumor-reactive expanded CD4+ and CD8+ T-cells.
Conclusions: Treatment of relapsed cHL with P- GVD resulted in durable remissions in patients undergoing AHCT. Distinct patterns of peripheral blood T-cell expansion were observed based on MHC-I and MHC-II expression on tumor cells, with CD4+ T-cell clonal expansion uniquely expanding in patients with MHC-I-deficient tumors. Further analysis of the impact of P-GVD on T-cell differentiation and function is ongoing.
AcknowledgementT: Supported in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme LLC. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme LLC.
Disclosures
Shah:Amgen: Research Funding; ArcellX: Other: DSMB; Beyond Spring: Research Funding; BMS: Research Funding; Janssen: Research Funding. Schoder:Aileron Therapeutics: Honoraria. Yahalom:Convergent R.N.R Ltd.: Other: Provision of Services (uncompensated). Kumar:Beigene: Research Funding; Abbvie Pharmaceuticals: Research Funding; Adaptive Biotechnologies: Research Funding; Janssen: Consultancy; BridgeBio: Current equity holder in publicly-traded company; Loxo/Lily Oncology: Consultancy, Research Funding; Astra Zeneca: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Celgene: Research Funding; Kite Pharma: Consultancy; Pharmacyclics: Research Funding; Seattle Genetics: Research Funding. Falchi:Genmab: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Abbvie: Consultancy, Other: Advisory Board, Research Funding; Genentech: Consultancy, Other: Advisory Board, Research Funding; Seagen: Other: Advisory Board; ADC Therapeutics: Other: Advisory Board; AstraZeneca: Consultancy. Hamlin:ADC Therapeutics: Consultancy. Palomba:GarudaTherapeutics: Honoraria; Novartis: Honoraria; Pluto Immunotherapeutics: Honoraria; Rheos: Honoraria; Seres Therapeutics: Honoraria, Patents & Royalties; Smart Immune: Honoraria; Thymofox: Honoraria; Synthekine: Honoraria; Ceramedix: Honoraria; Kite: Honoraria; Juno: Honoraria, Patents & Royalties; MustangBio: Honoraria; Cellectar: Honoraria; BMS: Honoraria. Johnson:Myeloid Therapeutics: Consultancy. Zelenetz:None other than mutual funds (401K): Current equity holder in publicly-traded company; SAB: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Lymphoma Research Foundation: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; MEI Pharma Inc: Consultancy, Honoraria, Research Funding; Abbvie: Research Funding; Gilead: Consultancy, Honoraria; Janssen Pharmaceuticals: Consultancy, Honoraria. Salles:BeiGene: Consultancy; ATB Therapeutics: Consultancy; BMS/Celgene: Consultancy; AbbVie: Consultancy, Honoraria; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Molecular Partners: Consultancy; Merck: Consultancy, Honoraria; Ipsen: Consultancy, Research Funding; Debiopharm: Consultancy; Nurix: Consultancy; EPIZYME: Consultancy; Orna: Consultancy; Loxo/Lilly: Consultancy; Kite/Gilead: Consultancy; Janssen: Consultancy, Research Funding; Genmab: Consultancy; Incyte: Consultancy; Nordic Nanovector: Consultancy; Owkin: Current holder of stock options in a privately-held company; Novartis: Consultancy. Vardhana:Immunai: Consultancy; Koch Disruptive Technologies: Consultancy. Moskowitz:Seattle Genetics: Honoraria, Research Funding; Merck: Honoraria, Research Funding; Incyte: Research Funding; Bristol-Myers Squibb: Research Funding; Beigene: Research Funding; ADC Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal